The primary focus of our research is to develop and demonstrate the utility of ‘time-resolved flow cytometry.’ We build, modify, and advance a variety of flow cytometry systems to enable the measurement of the fluorescence lifetime, as a unique fluorescence decay-dependent parameter. Our research stemmed from work conducted by Dr. Houston when she was a Director’s Postdoctoral Fellow at the Los Alamos National Laboratory (LANL) from 2006-2009. In the late 1980s “phase-sensitive flow cytometry” was established at LANL as a new tool for counting cells based on the fluorescence lifetime parameter.
During those early years establishing lifetime technologies, two ‘phase’ instruments were built by a group of LANL cytometrists & scientists (including but not limited to: J. Steinkamp, H. Crissman, L. Sklar, T. Yoshida, C. Deka, J. Keij, S. Cram, J. Jett, R. Habbersett, & J. Martin). Both lifetime cytometers were used to study many new cell applications and initiated many new collaborations. Around 2002, one of the phase instruments in Los Alamos was disassembled and the other remained in tact but was not used owing to a shift in the interest of cytometry instrumentation development.
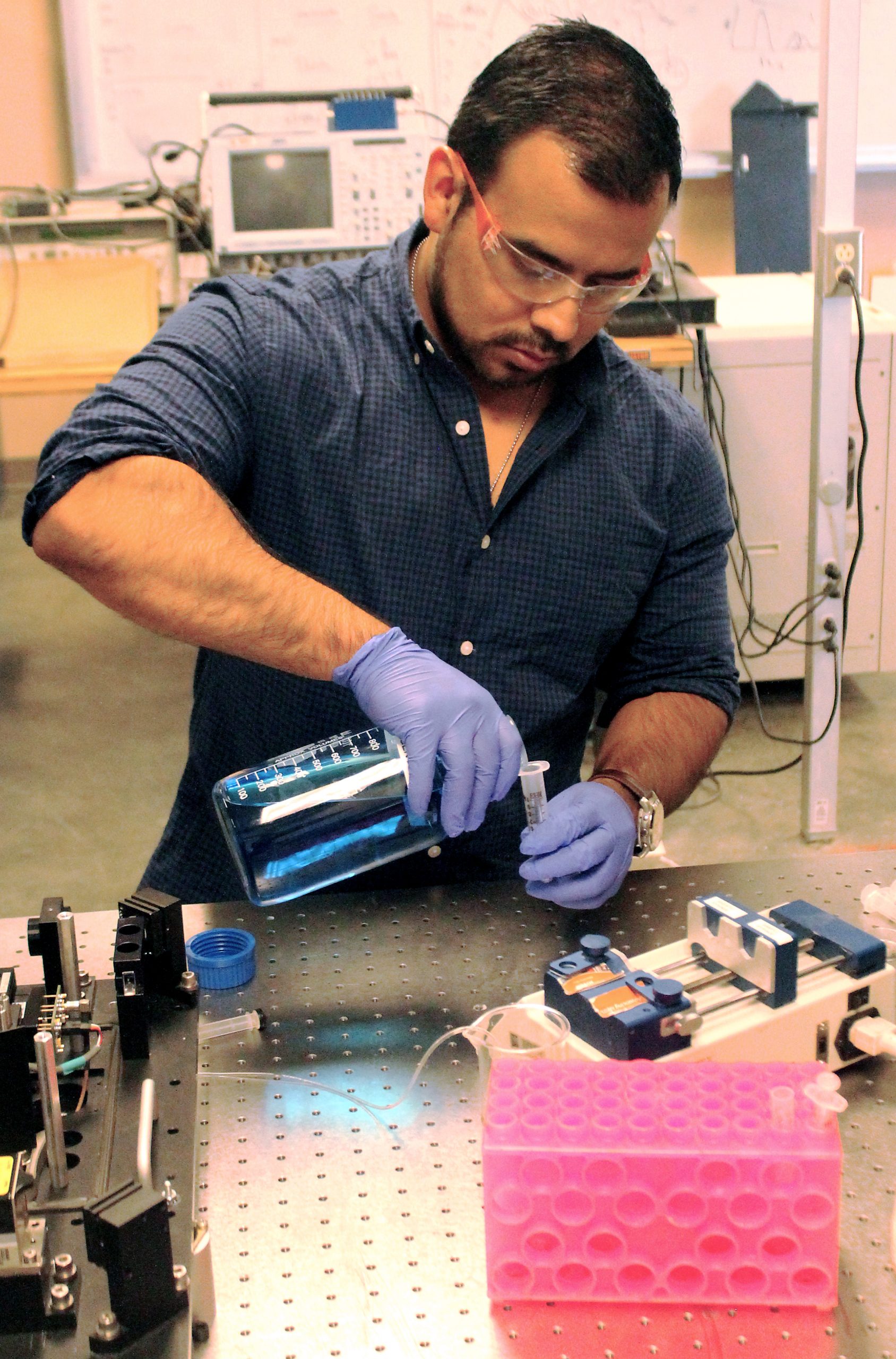
NMSU chemical engineering Ph.D. candidate Jesse Sambrano received a National Institute of Health grant supported by the National Institute of Biomedical Imaging and Bioengineering. (NMSU photo by Billy Huntsman) OCT16
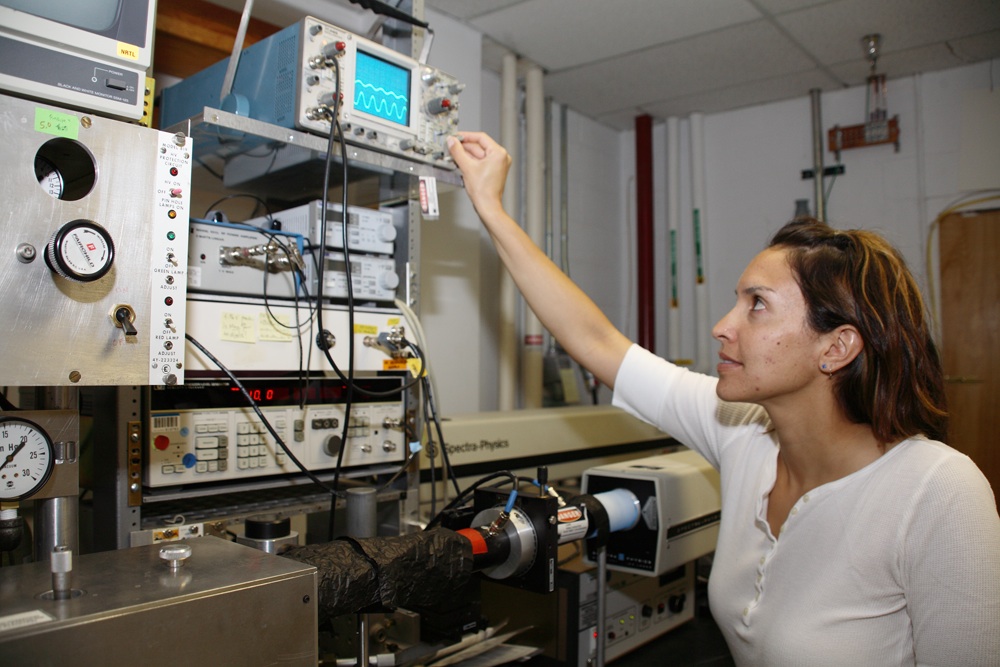
New and exciting areas for cytometry at LANL were expanding (acoustic focusing, full spectral cytometry, high sensitivity systems, low-cost portable systems, etc). At that time, Dr. Houston began to introduce new techniques that would advance how lifetime measurements are made in cytometry (see picture to the right of Dr. Houston circa 2006). Under the direction of the National Flow Cytometry Reasearch Resouce at LANL (led by Dr. James P. Freyer), Dr. Houston developed lifetime-based cell sorting and helped to advance digital techniques for measuring fluorescence lifetimes of cells with a cytometer.
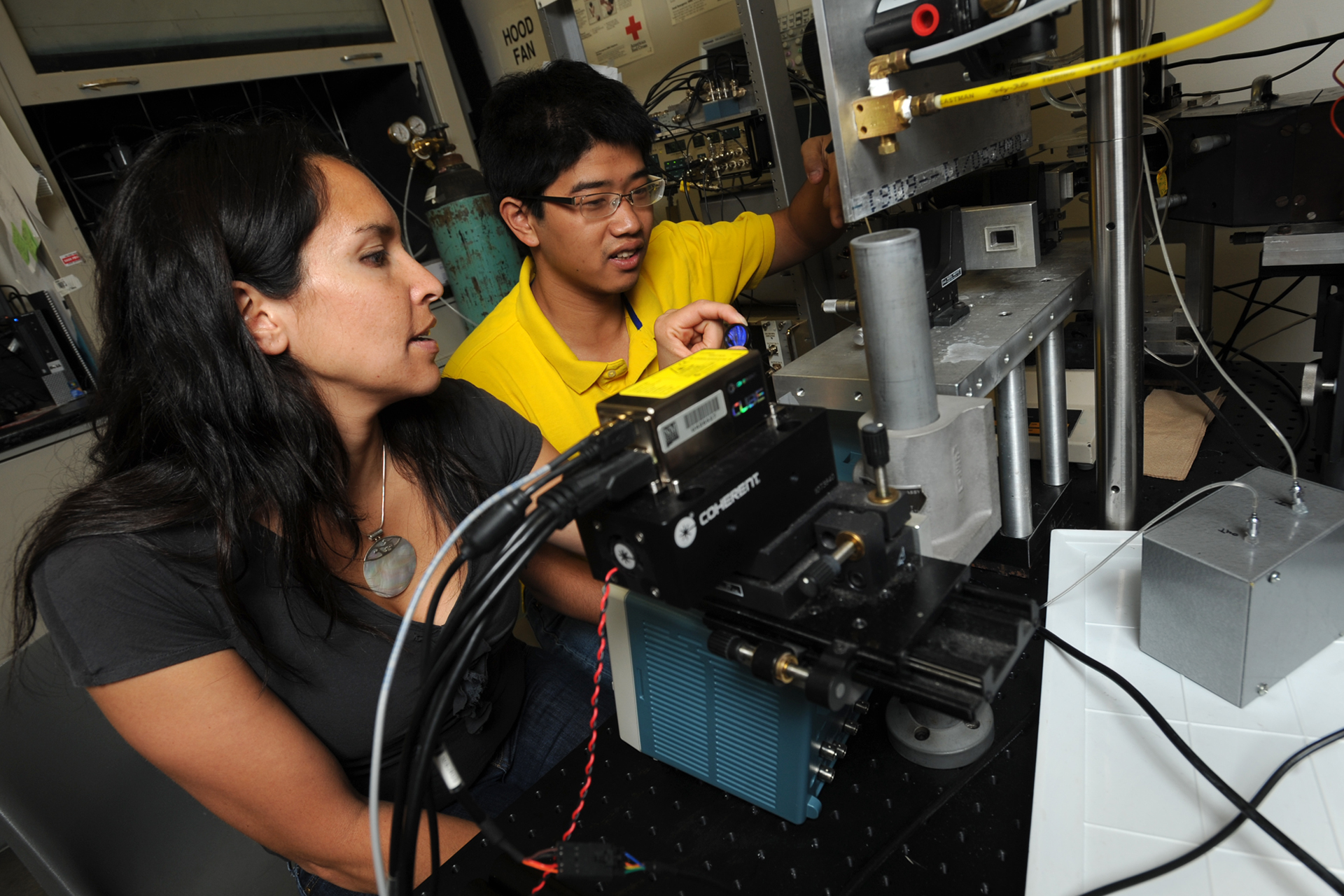
Around 2010, parts of the original LANL phase instrument were relocated to NMSU, when Dr. Houston began a faculty appointment within the Department of Chemical & Materials Engineering. Since then, the Houston laboratory has transformed a number of lifetime technologies and in doing so, provided a mechanism for undergraduate and graduate students at NMSU access to cross-disciplinary biomedical engineering research (photos include: Jesse Sambrano, Ph.D., CHME 2018, Matthew Mena undergraduate 2016, Ruofan Cao, Ph.D. CHME 2015, and Patrick Jenkins, M.S. CHME 2012).
Flow Cytometry and Fluorescence Lifetime Analysis
Flow cytometry is a method of counting, analyzing, and sorting individual cells and particles at the high-throughput level. Standard cytometry instruments detect individual cells (or small particles) as they pass in single file through a tightly focused laser beam in the plane of an optical detector. Fluorescence or Raleigh scatter are the primary optical signals that make up “multiparametric” measurements in cytometric analyses.
Our lab has a long-standing interest in improving the ability of flow cytometry systems to measure the fluorescence lifetime, or average time a fluorophore has spent in its excited state prior to relaxation to the ground state.

This is known as fluorescence decay kinetics and is appealing to the cytometry community because it can provide additional quantitative information about the fluorophore under interrogation or molecular milieu to which the fluorophore is attached. For example, the fluorescence lifetime is independent of fluorophore concentration and can help discriminate signals that have similar emission strength and which spectrally overlap.

Our laboratory continues to pioneer new lifetime techniques to make sensitive fluorescence lifetime measurements using ‘frequency-domain’ and ‘time-domain’ methods of analysis. We are specifically studying a variety of biomedical and health-related applications such as (but not limited to): (i) time-dependent methods for detecting autofluorescence; (ii) sorting of cells expressing fluorescence proteins based on fluorescence lifetime; (iii) phase filtering techniques to identify the uniqueness of fluorescent tags that occur in a “dual-labeled” fashion on the surface or interior of cells; (iv) multiplexing with materials exhibiting multiple lifetimes; and (v) heterodyning/multi-frequency measurements for multiexponential fluorescence decay.

10/16/2015: NMSU chemical engineering professor Jessica Houston, left, and NMSU biochemistry professor Kevin Houston demonstrate how they use their custom-built flow cytometer to evaluate cancer cell cultures. Jessica Houston and her research team developed the flow cytometer in her lab, which in turn is used by Kevin Houston to conduct research in his cancer cell biochemistry lab. (Photo by Darren Phillips)
May of our applications require novelty cytometry systems and instrumentation. Some examples of the new techniques we have introduced include: (1) the measurement of multi-exponential fluorescence decay kinetics in flow with digital data acquisition techniques; (2) cell sorting based on fluorescence lifetimes; (3) phase-filtering techniques, and (4) non-modulated laser measurement of lifetimes.


